
Image: Top 10 Largest Flying Dinosaurs (YouTube video)
By R. J. L.
Millions of years ago the atmosphere was dense and moist, the thick air made it possible for even very big birds and dinosaurs to fly. Some of the flying dinosaurs would not be able to fly if they were alive in todays small, thin and dry atmosphere.
“Dinosaurs that roamed the Earth 250 million years ago knew a world with five times more carbon dioxide than is present on Earth today, researchers say, and new techniques for estimating the amount of carbon dioxide on prehistoric Earth may help scientists predict how Earth’s climate may change in the future.
The findings are detailed in a recent paper published in the journal Proceedings of the National Academy of Sciences.
During the Jurassic Period, dinosaurs — ranging from the plant-eating Diplodocus and Brachiosaurus to the meat-craving Ceratosaurus and Megalosaurus — ruled the world. During this time, the Earth’s interior was not standing still; rather, the supercontinent Pangaea had started to split into two smaller landmasses, called Laurasia and Gondwana.
These tectonic movements made the oceans close up and the tectonic plates sink into the Earth. This process, called subduction,led to volcanism at the surface, with rocks constantly melting and emitting CO2 into the atmosphere. Huge amounts of this greenhouse gas made the climate during the Jurassic Period extremely humid and warm, said geoscientist Douwe van der Meer, lead author of the study and a researcher at Utrecht University in the Netherlands. [Weather vs. Climate: Test Yourself]
Scientists have known for some time that a large amount of volcanic activity results in more CO2 than is present on Earth today, but with previous methods, it had been tricky to come up with a reliable estimate.
Looking deep inside
Van der Meer’s team used a cutting-edge imaging technique called seismic tomography to reconstruct 250 million years of volcanic CO2 emissions.
To do so, the researchers analyzed earthquake waves traveling through Earth, to image the structure of the Earth’s interior.
“This method is comparable to CT scans used in hospitals to image inside bodies,” van der Meer said. “With sufficient earthquake wave travel times, one can create a velocity model of the Earth. Faster regions are more dense, colder material plates that sunk into the Earth.”
The aim has been to demonstrate how variations in plate tectonics have led to variations in CO2 emissions from volcanoes 250 million years ago.
And the deeper the imaging equipment goes, the farther back in time scientists can see — as far back as 250 million years, said van der Meer. “Essentially, we can see the breakup of the supercontinent Pangaea, and the opening and closing of oceans,” he said.
In other words, the scans depicted the interior of the Earth, enabling the researchers to “see” the tectonic plates that have sunk into the planet over the past 250 million years.
The researchers then quantified the plates that have sunk into the deep Earth, and their calculations showed that the Earth produced twice as much CO2 as there is today.
The scientists then inserted this number into a comprehensive, commonly used paleoclimate model, to calculate how all the volcanic CO2 emissions at the time would have added up. Because there was also less CO2 being removed from the atmosphere by vegetation and by weathering rocks than today, total atmospheric CO2 levels were probably five times higher than at the present, the researchers said.
The findings suggest much higher CO2 levels than had been estimated in previous studies conducted in the 1980s and 1990s. More of this study …
History of Oxygen and Ozone
Earth is believed to have formed about 5 billion years ago. In the first 500 million years a dense atmosphere emerged from the vapor and gases that were expelled during degassing of the planet’s interior. These gases may have consisted of hydrogen (H2), water vapor, methane (CH4) , and carbon oxides. Prior to 3.5 billion years ago the atmosphere probably consisted of carbon dioxide (CO2), carbon monoxide (CO), water (H2O), nitrogen (N2), and hydrogen.
The hydrosphere was formed 4 billion years ago from the condensation of water vapor, resulting in oceans of water in which sedimentation occured.
The most important feature of the ancient environment was the absence of free oxygen. Evidence of such an anaerobic reducing atmosphere is hidden in early rock formations that contain many elements, such as iron and uranium, in their reduced states. Elements in this state are not found in the rocks of mid-Precambrian and younger ages, less than 3 billion years old.
One billion years ago, early aquatic organisms called blue-green algae began using energy from the Sun to split molecules of H2O and CO2 and recombine them into organic compounds and molecular oxygen (O2). This solar energy conversion process is known as photosynthesis. Some of the photosynthetically created oxygen combined with organic carbon to recreate CO2 molecules. The remaining oxygen accumulated in the atmosphere, touching off a massive ecological disaster with respect to early existing anaerobic organisms. As oxygen in the atmosphere increased, CO2 decreased.
High in the atmosphere, some oxygen (O2) molecules absorbed energy from the Sun’s ultraviolet (UV) rays and split to form single oxygen atoms. These atoms combining with remaining oxygen (O2) to form ozone (O3) molecules, which are very effective at absorbing UV rays. The thin layer of ozone that surrounds Earth acts as a shield, protecting the planet from irradiation by UV light.
The amount of ozone required to shield Earth from biologically lethal UV radiation, wavelengths from 200 to 300 nanometers (nm), is believed to have been in existence 600 million years ago. At this time, the oxygen level was approximately 10% of its present atmospheric concentration. Prior to this period, life was restricted to the ocean. The presence of ozone enabled organisms to develop and live on the land. Ozone played a significant role in the evolution of life on Earth, and allows life as we presently know it to exist. Ref.: http://teachertech.rice.edu/Participants/louviere/history.html
If the atmosphere had (at least) 4 times as much Carbon Dioxide (CO2) in the atmosphere than today and the Oxygen level was 32% (today 21%), the whole atmosphere had to be larger, and more dense.
……..
The Early Ultraviolet Problem
The genetic materials of cells (DNA) is highly susceptible to damage by ultraviolet light at wavelengths near 0.25 µm. It is estimated that typical contemporary microorganisms would be killed in a matter of seconds if exposed to the full intensity of solar radiation at these wavelength. Today, of course, such organisms are protected by the atmospheric ozone layer that effectively absorbs light at these short wavelengths, but what happened in the early Earth prior to the significant production of atmospheric oxygen? There is no problem for the original non-photosynthetic microorganisms that could quite happily have lived in the deep ocean and in muds, well hidden from sunlight. But for the early photosynthetic prokaryotes, it must have been a matter of life and death. More …
……..
CARBON DIOXIDE
There are two gases in the earth’s atmosphere without which living organisms could not exist.
Oxygen is the most abundant, 21% by volume, but without carbon dioxide, which is currently only about 0.04 percent (400ppm) by volume, both the oxygen itself, and most living organisms on earth could not exist at all.
This happened when the more complex of the two living cells (called “eukaryote”) evolved a process called a “chloroplast” some 3 billion years ago, which utilized a chemical called chlorophyll to capture energy from the sun and convert carbon dioxide and nitrogen into a range of chemical compounds and structural polymers by photosynthesis. These substances provide all the food required by the organisms not endowed with a chloroplast organelle in their cells.
This process also produced all of the oxygen in the atmosphere
The relative proportions of carbon dioxide and oxygen have varied very widely over the geological ages.


It will be seen that there is no correlation whatsoever between carbon dioxide concentration and the temperature at the earth’s surface.
During the latter part of the Carboniferous, the Permian and the first half of the Triassic period, 250-320 million years ago, carbon dioxide concentration was half what it is today but the temperature was 10ºC higher than today . Oxygen in the atmosphere fluctuated from 15 to 35% during this period
From the Cretaceous to the Eocene 35 to 100 million years ago, a high temperature went with declining carbon dioxide.
The theory that carbon dioxide concentration is related to the temperature of the earth’s surface is therefore wrong.
The growth of plants in the Carboniferous caused a reduction in atmospheric oxygen and carbon dioxide, forming the basis for large deposits of dead plants and other organisms. Plant debris became the basis for peat and coal., smaller organisms provided oil and gas, both after millions of years of applied heat and pressure from geological change; mountain building, erosion, deposition of sediments, volcanic eruptions, rises and fall of sea level and movement of continents. Marine organisms used carbon dioxide to build shells and coral polyps and these became the basis of limestone rocks. More ..
………..
The Thick Mesozoic Atmosphere
It may be hard to imagine that the Earth’s air could be so thick that its density would be comparable to water. Nevertheless, there is no reason why a gas can not be compressed so much that it has properties similar to that of a liquid, and in fact compressing a gas into a liquid is a common industrial process.
In order to compress the air near the Earth’s surface, there has to be a substantial amount of overlapping air pressing down on the ground level air. Thus the high density ground level air is evidence of an extremely thick Mesozoic atmosphere.
Unlike water or other liquids that have nearly constant density between the top and the bottom, the density of a planet’s atmosphere increases as one travels from the darkness of space downward to the planet’s surface. In addition, there is also an increase in pressure as we move downward towards the surface. Close to the planet’s surface both the atmosphere’s density and the atmospheric pressure is the greatest due to the weight of all of the air above compressing the air at the surface. More …
…….
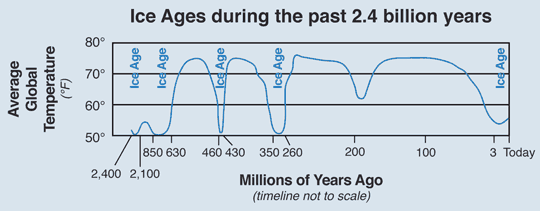
What caused the temperature to change so much in the geological past?
Different factors as cloud cover, cosmic rays, Milankovitch cycles and variation in the sun over time.
As we know, the oceans cover 70 – 71% of the earth’s surface, ocean currents is very important for local and regional weather, but also the earth’s climate. Water carry 90% of all the energy the earth is recieving from the sun.
How these currents flow and if, how much and where, seems to be very important.
Continental drift, how the landmasses on earth are located and what kind of ocean currents they allow would greatly influence the temperature. It seems that if such currents are disrupted, changed, increased or decreased – it has the potential to change the temperature observed in the records.
Dr. Ptrick Moore – “.. this would be a dead planet.”
………..
A lower Carbon Dioxide level caused by all natural processes taking Carbon Dioxide (CO2) out of the atmosphere, burying it in the ground as limestone etc. will also mean less Oxygen.
If you take out all CO2 and Oxygen, the rest of the lifeless atmosphere would be 21% smaller and less dense. Airplanes would have problems flying and there would be no more Oxygen to produce Ozone to protect us from UV radiation from the sun. We’re talking a planetary big Ozone-hole.
For environmentalists (the “greens”) to wage a war on Carbon Dioxide (CO2) seem to be complete madness. The process of taking CO2 out of the atmosphere is going on all the time, all natural. When we reach the level when plants no longer grow (at 180 ppmv.) it’s probably too late ..
To secure a healthy and safe level of CO2., if even possible, would be time consuming, there is no time to play around with “green” nutcases, this extremely expensive madness needs to stop.
……….
References


